Abstract
Introduction: Previous studies indicate that follicular lymphoma patients who have progression of disease (POD) within 24 months (ie POD24) of receiving frontline chemoimmunotherapy have worse overall survival and thus constitute a high-risk population (Casulo et al. J Clin Oncol 33:2516-2522, 2015; Jurinovic et al., Blood 128:1112-1120, 2016). We have previously reported in the CHRONOS-1 study in patients with relapsed or refractory indolent B-cell lymphoma that treatment with the pan-class I phosphatidylinositol 3-kinase (PI3K) inhibitor copanlisib resulted in durable responses with a manageable safety profile (Dreyling et al., J Clin Oncol 35:3898-3905, 2017). We explore here the outcomes for the subset of patients with rapid POD from the CHRONOS-1 study.
Methods: Patients with histologically confirmed indolent B-cell non-Hodgkin lymphoma and relapsed after, or refractory to, ≥2 prior lines of treatment were eligible. Previous treatment had to include rituximab (R) and an alkylating agent or regimen. Copanlisib was administered at a fixed dose of 60 mg via 1-hour I.V. infusion on days 1, 8 and 15 of a 28-day cycle. Treatment continued until progression or unacceptable toxicity. The primary efficacy endpoint was objective response rate after ≥4 cycles as assessed per independent radiologic review (Cheson et al., JCO 20:579, 2007). Secondary efficacy endpoints included duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Adverse events (AEs) were reported using MedDRA (version 19.1). This exploratory analysis was based on patient's first-line treatment.
Results: A total of 140 patients were treated and evaluable for POD based on first-line treatment; the principal histologies were follicular lymphoma (FL; n=102) and marginal zone lymphoma (n=23). First-line treatments in 140 evaluable patients included: 34% R-CHOP, 23% R-other, 21% R-CVP, 18% chemotherapy, and 2% patients R only. For FL patients, 87 of 102 (85%) received some form of R-chemotherapy as first-line treatment.
A total of 93 patients (66.4%) progressed in less than 24 months and were deemed the POD<24 group for this analysis; the remaining 47 patients (33.6%) were classified as POD>24. The median time from 1st line treatment to first POD was 11.0 months in the POD<24 and 35.3 months in the POD>24 group. The median number of lines of prior therapy for both groups was 3. The median time to progression for the most recent line of therapy prior to start of copanlisib treatment was also shorter for the POD<24 group (7.0 months; 65.6% refractory) than for the POD>24 group (15.7 months; 48.9% refractory).
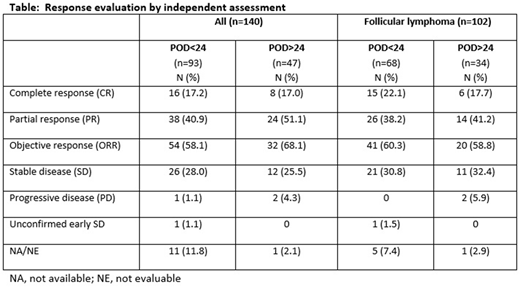
At the time of data cutoff (February 2018), the median duration of copanlisib treatment was 6.0 months (range 0.2-44.2) for the POD<24 group and 5.0 months (range 0.4-32.2) for POD>24. Whereas the fraction of patients with complete responses (CR) were identical in both groups (17%) [Table], the ORR was 58.1% in the POD<24 group and 68.1% in the POD>24 group. In FL patients the ORR was similar in both groups, but the percent of patients with CR was higher in the POD<24 group (22.1%) compared to the POD>24 group (17.7%). The overall median DOR was 14.9 months and 14.1 months, respectively. Median PFS was 11.3 months in the POD<24 group and 17.6 months in the POD>24 group; approximately 50% censored events in each group. Median OS was 42.6 months in the POD<24 group but had not yet been reached in the POD>24 group.
The median duration of safety follow-up was 6.7 months in the POD<24 group and 6.1 months in the POD>24 group. All-grade treatment-emergent adverse events (AEs) were similar in both groups, with grade (G) 3/4 events 48.4%/33.3% in the POD<24 group and 66.0%/21.3% in the POD>24 group. Serious AEs considered treatment-related occurred in 29.3% of patients (15.0% G3/7.1% G4). There were 3 treatment-related G5 events, 2 (2.2%) in the POD<24 group and 1 (2.1%) in the POD>24 group.
Conclusions: Two-thirds of patients treated with copanlisib in the CHRONOS-1 study were considered high-risk based on POD in less than 24 months after first-line therapy, yet the efficacy of copanlisib in both groups was similar. These results suggest that copanlisib treatment should be explored as treatment for patients failing to achieve durable responses in the first-line setting.
Leppa:Roche: Consultancy, Honoraria, Research Funding; Bayer: Research Funding; Takeda: Consultancy, Research Funding; Celgene: Consultancy; Janssen: Consultancy, Research Funding. Follows:Abbvie: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Lenz:Gilead: Consultancy, Honoraria; Celgene Corp.: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding, Speakers Bureau; Novartis: Research Funding; Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding, Speakers Bureau. Demeter:Amgen: Consultancy; BMS: Consultancy; Novartis: Consultancy; Aramis Pharma: Consultancy; Pfizer: Consultancy; Roche: Consultancy; Angelini: Consultancy. Rodrigues:Bayer: Employment. Wirtz:Bayer: Employment. Hiemeyer:Bayer: Employment. Liu:Bayer: Employment. Koechert:Bayer: Employment. Garcia-Vargas:Bayer: Employment. Childs:Bayer: Employment. Zinzani:PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Speakers Bureau; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees. Dreyling:Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal